In fall 1993, Arthur Horwich of the Yale School of Medicine was attending a conference in London when he decided to check in with his lab. Calling from a payphone on the street, he learned that his colleagues had made a breakthrough on their latest project. “I said, ‘Don’t tell me anything. I’m changing my airline reservation, and I’m flying home tonight,’” Horwich recalls.
The next day was “magical,” he says. Walking into the Yale lab of one of his collaborators, Horwich saw on a computer screen a colorful image that was the culmination of more than three years of work. The image depicted the crystal structure of the bacterial protein GroEL, which helps other proteins fold into the correct shapes. Previous research had indicated that GroEL consisted of two stacked rings, forming a hollow cylinder, but the crystal structure revealed the protein’s architecture in greater detail. “It was beautiful to see the rings at high resolution,” Horwich says.
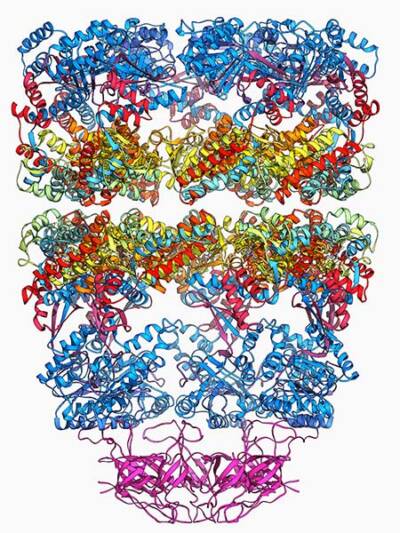
Molecular model showing the structure of a GroEL GroES (ADP)7 chaperonin complex
Courtesy of Laguna Design / Science Source
That close-up of GroEL, together with earlier electron microscope images, helped Horwich and his sometime collaborator Franz-Ulrich Hartl, now at the Max Planck Institute of Biochemistry, uncover a mechanism that allows some proteins to fold properly. Their research over several years revealed that GroEL and its counterparts, part of a family of proteins known as chaperonins, form structures that serve as cellular changing rooms. An unfolded protein enters one of the structures and, isolated from the hurly-burly of the cytoplasm, begins to take on a new shape. A few seconds later, out pops a freshly folded protein, ready for action. Human cells also get folding help from chaperonins that include Hsp60, the mammalian version of GroEL.
Most proteins need to fold into specific shapes to perform their jobs. Hartl and Horwich’s findings were “important from a fundamental point of view,” says molecular biologist Peter Lund of the University of Birmingham, who wasn’t connected to their research. Thanks to their findings, “we know more about protein folding and how cells regulate it.” For the discovery, Horwich and Hartl shared the 2011 Albert Lasker Basic Medical Research Award. They were the first of three pairs of scientists to earn Lasker Awards for work on protein folding. The second team illuminated a mechanism that enables cells to identify and deal with misshapen proteins, and the third pair developed a powerful artificial intelligence tool that can predict the final folded structures of almost all known proteins.
Joining Forces to Decipher Folding
A protein is born as a linear strand and then twists, curls, coils, and bends into the appropriate shape, or conformation. But that molecular gymnastics sometimes goes awry, producing a malformed protein that may not function correctly. Many cystic fibrosis patients, for instance, carry mutations that deform the protein CFTR, which helps control the body’s balance of water and salts in locations such as the lining of the lungs. The distorted version of CFTR can’t commute to its workplace, the cell membrane. Abnormally folded proteins also are harmful because they tend to stick together, forming clumps that are toxic to cells. Those protein aggregates are hallmarks of various illnesses, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.
“Proteins would rather be folded than not,” Lund says. But for many years the question of how proteins “know” what shape to assume puzzled researchers. Then Nobel laureate Christian Anfinsen and colleagues seemingly found the answer. In the 1950s and 1960s, the scientists forced proteins to unfold in the test tube and discovered that the molecules naturally refolded into their former shape. For nearly 30 years, researchers assumed that proteins also folded spontaneously in cells. “People thought that protein folding was solved. There was nothing more to discover,” Hartl says. However, “the cytoplasm is a very crowded environment,” he says. It is jam-packed with many kinds of proteins, and they can cause freshly synthesized proteins to bunch up instead of folding. When a protein is trying to fold, “the conditions are against it,” Lund says.

Horwich (left) and Hartl attending a party at Hartl’s apartment in Munich in 1991
Courtesy of Hartl
Hartl and Horwich stumbled onto one solution for the problem. Both had studied medicine but became enchanted with research. Horwich ended up working as a physician and a scientist, but Hartl opted not to practice medicine. “That’s a decision that saved many lives,” he quips. In 1988, both researchers were independently probing the same question—how proteins enter mitochondria, the cell’s energy-producing organelles. Other scientists had shown that proteins had to be in an unfolded state to slip into the organelles and then folded into their active form once inside.
Horwich and his team had identified a mutation in yeast that kept mitochondrial proteins from forming properly. But the researchers ran into practical difficulties when trying to uncover the underlying defect. “We were a tiny lab of only three people,” Horwich says. Then one day Horwich unexpectedly got a call from Hartl, who had just started his own research group at the University of Munich, and his boss. “They said, ‘we understand you are studying mutations of yeast that might affect proteins in the mitochondria. Could you use some help?’” Horwich recalls. He said yes.
“We collaborated intensely for a time,” Hartl says. The two researchers worked in each other’s labs, shared results, and batted around hypotheses. The 11 papers they wrote together over four years revealed that the yeast mutation disabled Hsp60, showed that Hsp60 folds proteins, and further described the folding mechanism. The scientists continued making discoveries about the mechanism after their collaboration ended, including Hartl’s finding that Hsp60 cooperates with another key protein, Hsp70, to ensure correct folding.
The researchers’ work, along with results from other scientists, shows that GroEL forms a protective chamber in which proteins can fold without interference from other molecules. A second component, known as GroES, serves as a lid on the chamber, ensuring that the protein inside can fold in peace. Hsp60 operates similarly, with the protein Hsp10 forming its lid. GroEL and Hsp60 stand out because, unlike traditional enzymes, they are “catalyzing not a chemical reaction but a conformational reaction,” says biochemist Jonathan Weissman of MIT, Horwich’s postdoc in the mid-1990s. “The idea that nature had something to help a lot of proteins fold is a big conceptual advance.”
Getting Abnormal Proteins Back into Shape
Hsp60 and GroEL aren’t the only folding sites in cells. About one-third of human proteins fold inside an organelle called the endoplasmic reticulum (ER), a network of membrane-enclosed tubes. Occasionally, however, those proteins don’t fold correctly. Two researchers—Kazutoshi Mori, now at Kyoto University, and Peter Walter, now at Altos Labs—shared the 2014 Albert Lasker Basic Medical Research Award for teasing out how cells cope with that situation.

Mori (left) and Walter at the 2014 Lasker Awards Ceremony
The ER handles proteins that the cell will secrete or install in its membrane. When a cell is under duress, those proteins can misfold and begin to build up, producing toxic aggregates or causing other problems. To combat those effects, cells instigate the unfolded protein response, a process that spurs abnormal proteins to refold or destroys them. “The unfolded protein response is very important,” says surgical oncologist Jonathan Harnoss of the University of Giessen. “It takes place in every cell in the body.” Because of its significance for cell survival, he adds, the response “plays a huge role” in a range of illnesses, including diabetes, cardiovascular disease, rheumatoid arthritis, and the disease he studies, cancer.
In the late 1980s, Joseph Sambrook and Mary-Jane Gething of the University of Texas Southwestern Medical Center and colleagues postulated that cells relied on the unfolded protein response when aberrant proteins began accumulating. “Walter and Mori put some flesh on this,” says molecular biologist David Ron of the University of Cambridge. “They said, ‘here is an example of how it works.’”
The two scientists converged on that area of research from different starting points. Mori, who says he became fascinated with science by reading comic books, earned a PhD in biochemistry at Kyoto University. After four years working as a cancer researcher, he left his native Japan in 1989 to became a postdoc in the labs of Sambrook and Gething at UT Southwestern. “I did not know even the importance of protein folding at that time,” Mori says. But soon he was delving into the unfolded protein response. Walter, a native of what was then West Germany, had earned a PhD in molecular biology at Rockefeller University, studying under Nobel laureate and 1993 Albert Lasker Basic Medical Research Award winner Günter Blobel. Walter had performed well-regarded research on how cells direct certain proteins to the ER. After starting his own lab at the University of California, San Francisco, he became interested in how misfolding in the ER spurred a cell to turn on genes that orchestrate the unfolded protein response. “We wanted to learn how information travels backwards from the ER to the nucleus,” he said in an interview.
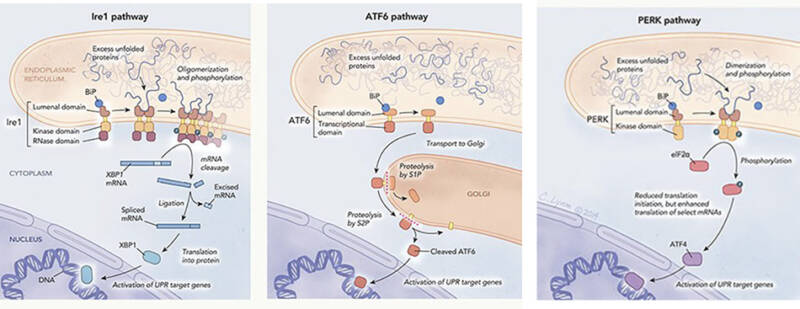
The unfolded protein response (UPR) is mediated by three pathways that originate in the endoplasmic reticulum: the Ire1 pathway (left), the the ATF6 pathway (middle) and the PERK pathway (right). Each one is named after the transmembrane protein (Ire1, ATF6, PERK) that interacts with unfolded proteins to initiate the UPR.
Illustration by Cassio Lynm
Though working independently, Walter and Mori ended up carrying out the same experiment, which involved scrutinizing mutant yeast colonies to identify cells with alterations that crippled the unfolded protein response. Those cells were white, whereas yeast with a functional response were blue. The work was laborious. To discover just three mutant cells, Mori spent six months examining 100,000 yeast colonies. But the effort paid off, allowing the researchers to zero in on the protein IRE1 as a key player in the unfolded protein response. “Their discovery of IRE1 enabled the whole field,” says biologist Martin Schroeder of Durham University, who had no connection to the work.
Mori’s and Walter’s teams published their results within three months of each other in 1993. Both scientists suspected that IRE1, which spans the ER membrane, sensed abnormally folded proteins in the organelle and somehow notified the cell’s nucleus. But neither man ever imagined how strange the communication mechanism would be.
To synthesize a particular protein, a cell first makes a messenger RNA (mRNA) copy of the gene that codes for the protein. The cell then edits the mRNA molecule, snipping out segments and rejoining the remaining pieces, a process known as splicing. Walter’s and Mori’s yeast studies showed that after detecting protein misfoldings in the ER, IRE1 spurs the cell to edit the mRNA that encodes another protein, Hac1. The cell then uses that rejiggered mRNA to make Hac1, which travels to the nucleus and switches on genes that trigger the unfolded protein response. The discovery, published in 1996 and 1997, challenged molecular biology orthodoxy. Researchers had thought that splicing occurred only in the nucleus. However, cells spliced Hac1’s mRNA in the cytoplasm. “Putting all that together at the mechanistic level is a very elegant and prize-deserving piece of work,” Ron says.
Mori and Walter continued to add to the knowledge about the unfolded protein response. In 1999, for instance, Mori and colleagues identified in mammalian cells a separate branch of the response that is initiated by the protein ATF6. Ron says the two scientists succeeded because “they were both there at the right time, they were the right age, they were well trained, they were clever, and they had attention to detail.” They also shared another equally important quality, he adds: They were “able to consider outlandish possibilities.”
AI Aces Protein Folding
Drug designers, vaccine developers, structural biologists, protein chemists, and evolutionary biologists are just some of the scientists who could benefit from the work of the winners of the 2023 Albert Lasker Basic Medical Research Award, Demis Hassabis and John Jumper of the London-based AI laboratory Google DeepMind. Hassabis, DeepMind’s co-founder and CEO, and Jumper, its director, led the team that developed AlphaFold, an AI system that can predict a protein’s structure with unparalleled accuracy. In 2024, the pair also shared the Nobel Prize in chemistry.
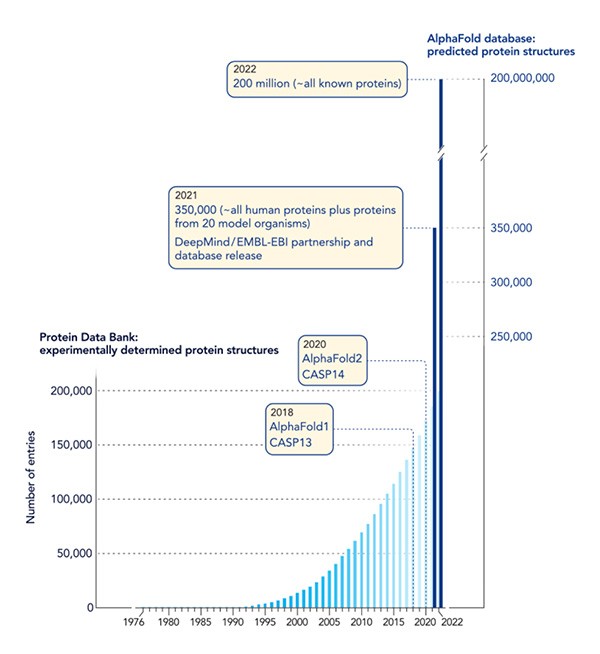
Graph showing how machine learning has catapulted the protein-structure field into a new realm. EMBL-EBI = European Molecular Biology Laboratory-European Bioinformatics Institute CASP = Critical Assessment of Structure Prediction
“They have revolutionized the field of structural biology and structural biochemistry,” says evolutionary structural biologist Christine Orengo of University College London. AlphaFold’s folding predictions could speed development of treatments, unlock ways to combat pollution, improve scientists’ understanding of how some proteins function, and spark various other advances. “It’s the biggest jump forward in AI in science,” says Marc Zimmer, a computational chemist at Connecticut College.
Scientists can experimentally determine the architecture of many proteins with techniques such as x-ray crystallography. But the work is slow, hard, and expensive. In more than 60 years of effort, researchers have published the structures of about 200,000 proteins, less than 1% of the total number that exists in nature. To fill the gap, for several decades scientists have tried to craft algorithms to predict proteins’ shapes solely from their amino acid sequences.
AlphaFold, which predicts proteins’ folded shapes by learning from experimentally determined structures, made its public debut in 2018 at the Critical Assessment of protein Structure Prediction (CASP), a protein folding showdown held every two years since 1994. CASP entrants try to produce the most accurate structure predictions for a set of proteins, with scores reflecting how closely results match actual shapes. AlphaFold stood out among the competitors, but its predictions still were not as accurate as experimentally determined results. “They were the best, but they weren’t over the moon,” says computational biologist and CASP co-founder John Moult of the University of Maryland.
At the 2020 CASP, however, “they blew everyone away,” Moult says. Predictions by DeepMind’s new version of the system, AlphaFold2, were virtually identical to structures determined by lab methods. Hassabis, Jumper, and their DeepMind colleagues incorporated “a multitude of ideas” to improve the system’s performance, as one review put it. For example, the team replaced the neural network—the analytical model that allows AlphaFold to learn—with a new, more powerful type also used by chatbots such as ChatGPT.
DeepMind has given other demonstrations of AlphaFold2’s prowess. The system predicted the structures of almost all roughly 24,000 human proteins and then did the same for almost every protein scientists have identified—some 200 million molecules. “In 2018, if anyone had gone to the protein chemists and said, ‘we will have the protein structures of every protein ever sequenced,’ they would have laughed at you,” Zimmer says. DeepMind also has introduced AlphaFold3, which can predict interactions among proteins, RNA, DNA, and other types of molecules. Many scientists are now using the versions of AlphaFold in their work. “It’s going to be transformative,” Orengo says.

Jumper (left) and Hassabis at the 2023 Lasker Awards Ceremony
The first drugs developed with help from AlphaFold have yet to reach clinical trials. Still, scientists are working to turn their deeper knowledge of folded protein structures into treatments. The first AI-designed drug entered clinical trials in 2023, for instance. A different trial is testing whether ramping up the unfolded protein response will shrink tumors in pancreatic cancer patients. And the Food and Drug Administration has approved a suite of what are known as correctors that spur faulty CFTR to refold into the correct shape in cystic fibrosis patients. “That’s a clear application of our understanding of protein folding,” Weissman says.
By Mitchell Leslie



